Development of Intranasal Insulin for Smoking Cessation
Description
Many cigarette smokers express a desire to quit smoking, but ~ 95% of cessation attempts fail.
Our lab develops new smoking cessation treatments. We recently completed studies in which we tested intranasal insulin for reducing cravings. We’ve designed the drug formulation protocol, secured NIH funding, ensured excellence in scientific, regulatory and technical aspects, and, ultimately, showed that intranasal insulin is safe and effective. Measured by Questionnaire of Smoking Urges, in two separate clinical trials, we’ve shown that the medication reduces nicotine cravings.
Figure 1
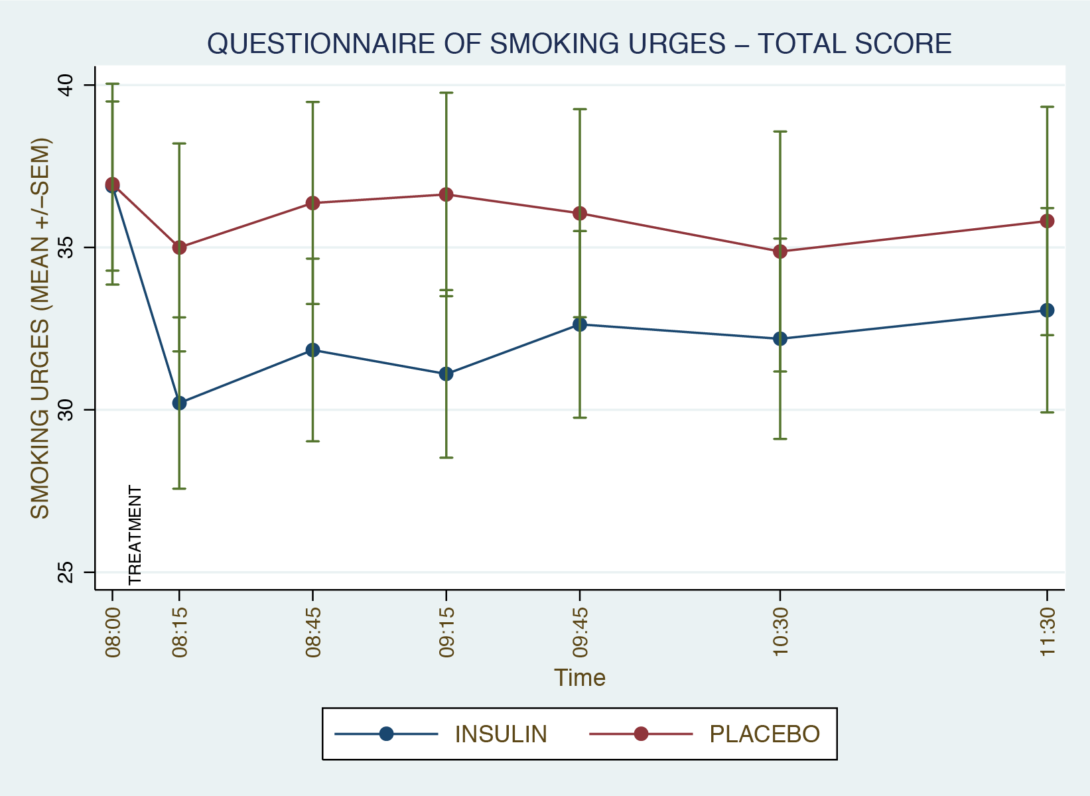
Figure 1. Original study nicotine craving ratings. The crossover study involved 36 hours of smoking abstinence with Questionnaire of Smoking Urges measurements taken on the morning of Day 2. Smoking urges decreased significantly with intranasal insulin compared to placebo (p ≤0.05). axis reflects clock time.
Figure 2
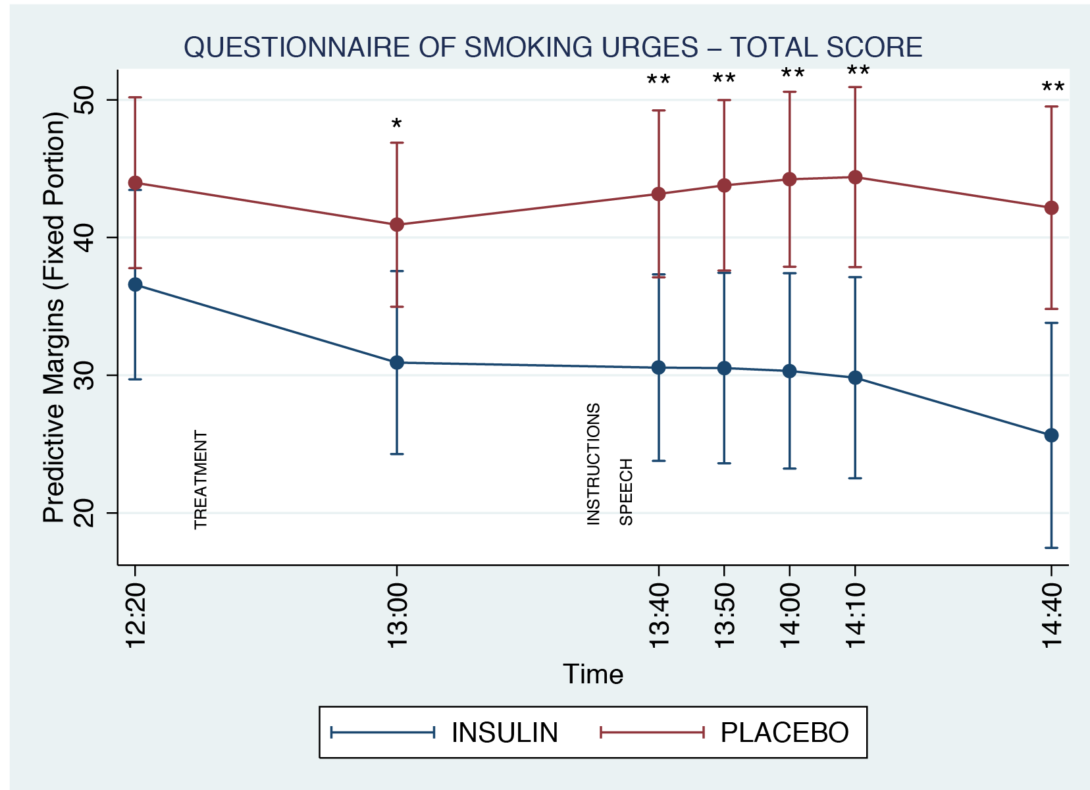
Figure 2. Replication/Extension study nicotine craving ratings during the course of study session. Smoking urges decreased significantly with intranasal insulin compared to placebo (p ≤0.05). The effect of intranasal insulin was detected following spray administration and lasted for 100 minutes through the stress task. “INSTRUCTIONS” and “SPEECH” on the graph below refer to the two parts of Trier Social Stress Test. “TREATMENT” refers to either insulin or placebo spray administration.
Reference
Reference
Hamidovic A, Khafaja M, Brandon V, Anderson J, Ray G, Allan AM, Burge MR. Reduction of smoking urges with intranasal insulin: a randomized, crossover, placebo-controlled clinical trial. Molecular Psychiatry. 2017 Oct;22(10):1413-1421. PubMed PMID: 28242873.